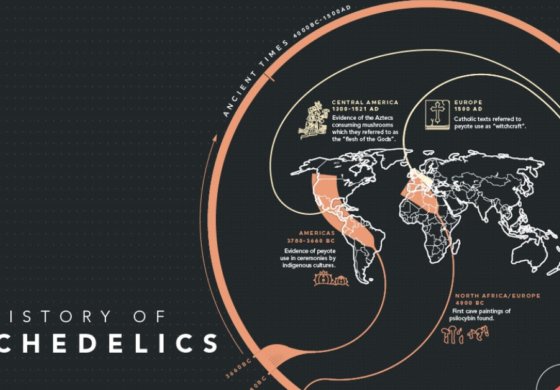
Revive Therapeutics Ltd. (CSE: RVV) (OTC: RVVTF) recently acquired the full rights to PharmaTher Inc.’s (CSE: PHRM) (OTC: PHRRF) intellectual property pertaining to psilocybin—the most popular psychedelic compound in clinical development worldwide.
The assets include preclinical research from the National Health Research Institutes in traumatic brain injury and stroke, as well as three key provisional patents with the U.S. Patent and Trademark Office. In aggregate, the assets were developed to target a U.S. FDA Orphan Drug Designation for psilocybin in traumatic brain injury and stroke.
“We are now in a position to advance our psilocybin program for future clinical development in various unmet medical needs in mental health, cancer and neurological diseases,” said Revive Therapeutics CEO Michael Frank.
Let’s take a look at how the acquisition fits in with the company’s unique strategy, the significant market that the asset target and why it’s such a synergistic acquisition.
Click here to receive an investor presentation and corporate updates
Unique Development Strategy
Revive Therapeutics’ long-term strategy has been to take advantage of the regulatory incentives at the FDA, including its Orphan Drug, Fast Track, Breakthrough Therapy and Rare Pediatric Disease designations to advance drugs through clinical trials. The acquisition of PharmaTher’s assets fit in with that model by targeting an Orphan Drug Designation.
The Orphan Drug Designation provides special status to a drug that is developed to treat a rare disease or condition. In exchange for developing the drug, the sponsor is eligible for tax credits worth 50% of the clinical drug testing cost and market exclusivity for seven years post approval—both lowering the cost of clinical trials and creating a barrier to entry.
Significant Unmet Medical Need
PharmaTher’s assets are also focused on large markets with significant unmet medical needs—or in other words, no effective treatment options for those suffering from the conditions.
The market for traumatic brain injury assessment and management devices reached nearly $3 billion in 2020, according to Grand View Research, and is expected to grow at an 8.3% compound annual growth rate through 2028. The CDC estimates that TBI is responsible for 2.8 million accidents and emergency visits each year in the U.S. with few treatment options.
Despite improved assessment and management technologies, there are few effective treatment options for TBI after it occurs, leaving sufferers with impaired thinking, memory, movement, sensation or emotional function. Between 2006 and 2014, the CDC found a 53% increase in emergency room visits, hospitalizations and deaths from TBI.
Revive Therapeutics anticipates initial research results from its psilocybin for TBI program during the first quarter of the year before submitting an FDA IND for psilocybin in TBI and beginning formal clinical trials—and PharmaTher’s assets will be instrumental.
Stroke represents a much larger market at about $30 billion in 2019, according to GM Insights, while a rising geriatric population is expected to drive 6.3% compound annual growth through 2026. Despite advances in identifying strokes and limiting the damage, there are few treatment options for those that suffer from the effects of a stroke.
Click here to receive an investor presentation and corporate updates
Synergistic Acquisition
The PharmaTher acquisition complements Revive Therapeutics’ existing psychedelics pharmaceutical platform that includes the development of an oral thin film product, a novel biosynthesis version of psilocybin, a clinical study evaluating psilocybin for methamphetamine use disorder and research in stroke, TBI and cancer.
With an innovative pathway to market and unique delivery systems, the company is well-positioned to unlock value for shareholders over time. Investors may want to keep a close eye on the stock over the coming months as it integrates the PharmaTher assets and advances various programs into clinical development.
For more information, visit the company’s website or download their investor presentation.
Click here to receive an investor presentation and corporate updates
This article was published by CFN Enterprises Inc. (OTCQB: CNFN), owner and operator of CFN Media, the industry’s leading agency and digital financial media network dedicated to the burgeoning CBD and legal cannabis industries. Call +1 (833) 420-CNFN for more information.